Ammonium Chloride and Sodium Hydroxide Net Ionic Equation
Net Ionic Equation N4HClNaOHNaCls NH 3 H 2 O. Type of Chemical Reaction.

How To Write The Net Ionic Equation For Nh3 Hcl Nh4cl Youtube
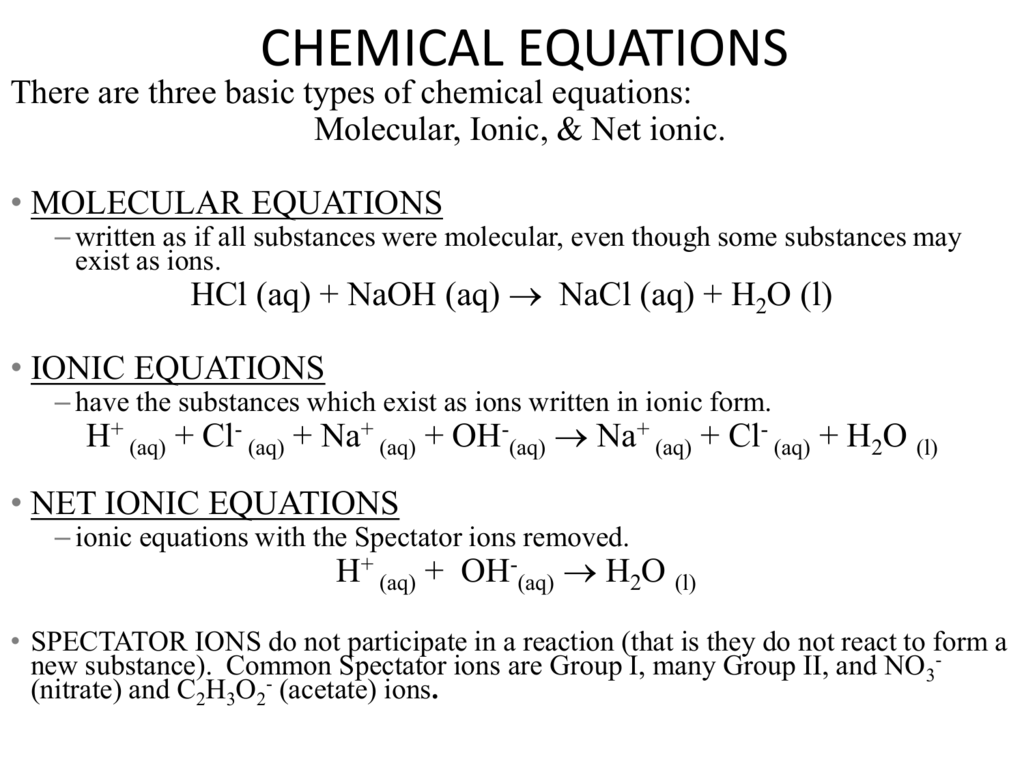
Separate the following balanced chemical equation into its total ionic equation.

. NH 4 Cl NaOH NaCl NH 3 H 2 O. Check the balance Ammonium chloride react with sodium hydroxide to produce sodium chloride ammonia and water. Any ammonium salt on reaction with an alkali would liberate ammonia gas.
Honors Chemistry Name_____ Period_____ Net Ionic Equation Worksheet READ THIS. Use H3O instead of H Chem. In this reaction Ammonium chloride and Sodium hydroxide are reacting to form Ammonia Water and Sodium chloride.
First we balance t. Ammonium chloride Sodium hydroxide Ammonia Water Sodium chloride. Answer 1 of 3.
Sodium hydroxide aq ammonium chloride aq Balanced Formula Equation. NH4Claq NaOHaq NH3g NaClaq H2Ol Every molecule above with an aq next to it is in aqueous solution and exists in the solution as its constituent ions. Net Ionic Equation NaClNaCl.
Science Chemistry Neutralization chemistry. For the best answers search on this site shorturlimavbz6. Write the net ionic equation for the equilibrium that is established when ammonium nitrate is dissolved in water.
Ammonium chloride Sodium hydroxide Observation Precipitate Gas Molecular Equation NH 4 Claq NaOHaqNaCls NH3gH 2 Ol Ionic equation N4HClNaOHNaCls NH 3 H 2 O. The full ionic equation shows all ionic species in the reaction. Write the net ionic equation for the reaction of calcium nitrate and sodium phosphate.
Ammonium Chloride Sodium Hydroxide NH_4Cl NaOH NH_3 H_2O NaCl There are so many chemical reactions that occur around us that we classify them into different types. For this reaction we have a chemical reaction. Assuming you were asking for the equati.
NH4NO3 NaOH NaNO3 NH3 H2O Here both the reactants NH4NO3 and NaOH are ionic compounds and they always exist as NH4 NO3 - ions and Na and OH-. It is an instance of a strong base displacing a weaker base from the latters salt. When a solution of sodium hydroxide is added to a solution of ammonium carbonate H 2 O is formed and ammonia gas NH 3 is released when the solution is heated.
When two solutions of ionic compounds are mixed a solid may form. 1 NaOH HCl NaClH2O. This reaction is classified as Strong Acid Strong Base Weak Acid Strong Base Strong Acid Weak Base Weak Acid Weak Base The extent of this reaction is Below 50 50 Above 50 100.
In the complete ionic equation soluble ionic compounds and strong acids are rewritten as dissociated ions. H2O NH3 NaCl The net ionic equation is NH4 OH- --. First of all you did not state the anion of the ammonium salt which actually doesnt matter because it will get cancelled out in the ionic equation but still you should mention.
What is the balanced net ionic equation for copper2chloride and potassium carbonate. Cd2 S2- ------ CdS s Widget. 2 NaOH NH4Cl NH3 H2O NaCl.
What is the balanced net ionic equation. Ammonium is an ion not to be confused with ammonia which is a gas. Cd2 2Cl- 2Na S2- --------- CdS s 2Na 2Cl- Net ionic equation all aq except CdS.
This type of reaction is called a precipitation reaction and the solid produced in the reaction is known as the precipitateYou can predict whether a precipitate will form using a list of solubility rules such as those found in the. Ammonium chloride will tend to react with sodium hydroxide to form ammonia water and sodium chloride. CdCl2 aq Na2S aq ------- CdS s 2NaCl aq Total ionic equation all aq except CdS.
The reactants for the molecular equation are these. H2O NH3 NaCl The net ionic equation is NH4 OH- --. There are three main steps for writing the net ionic equation for NH4Cl NaOH NaCl H2O NH3 Ammonium chloride Sodium hydroxide.
NH 4 Cl NaOH NH 3 H 2 O NaCl. CopperII chloride and leadII nitrate react in aqueous solutions by double replacement. Ammonium chloride and sodium hydroxide reaction net ionic equation.
Write the balanced chemical equation the overall ionic equation and the net ionic equa- tion for this reaction. 3 HCl NH3 H2O NH4Cl H2O. FeOH_ 3s darr NaCl In order to balance this chemical equation you can use the fact that the two reactants and sodium chloride are soluble in water which implies that they exist as ions in aqueous solution.
NH3 H2O. NH4Cl NaOH --. The conventional equation for the reaction is.
An aqueous solution of hydrochloric acid reacts with aqueous ammonia NH3 yielding aqueous ammonium chloride. Ammonium chloride will tend to react with sodium hydroxide to form ammonia water and sodium chloride. NH4NO3 NaOH NaNO3 NH3.
If 1027 g of copperII Chem. For ammonium nitrate sodium hydroxide Equation for the reaction is. NH 4 2 CO 3 aq NaOHaq ---.
Write the ionic and net ionic equation for this equation. Write a complete molecular complete ionic and net ionic equations for this reaction. NH4Cl NaOH --.
Ammonium chloride sodium hydroxide have only two ionic species each which is the case of ALL ionic compounds So the Full equation is simply NH4aq Cl-aq Naaq OH-aq which yield the exact same species because no precipitates gases or liquids are formed. Write the ionic and net ionic equation for this equation. Write the balanced NET IONIC equation for the reaction that occurs when ammonium chloride and sodium hydroxide are combined.
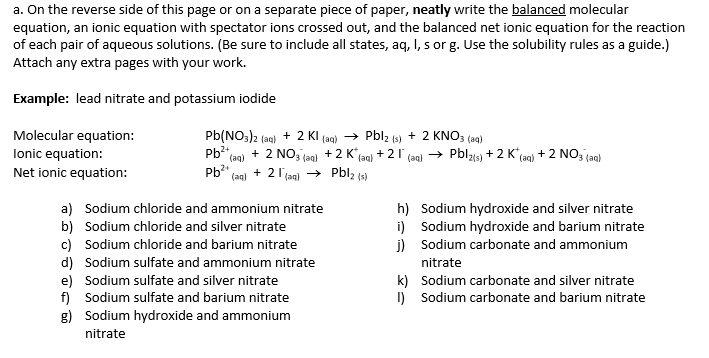
Solved On The Reverse Side Of This Page Or On A Separate Chegg Com

How To Write The Net Ionic Equation For Nh4cl Naoh Nacl H2o Nh3 Youtube
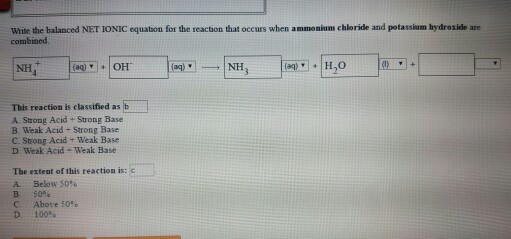
Solved Write The Balanced Net Ionic Equation For The Chegg Com

Solved Ammonium Chloride Sodium Hydroxide Observations Chegg Com

Solved Write The Balanced Net Ionic Equation For The Chegg Com

How To Write The Net Ionic Equation For Agno3 Nh4cl Agcl Nh4no3 Youtube

How To Write The Net Ionic Equation For Nh4no3 Naoh Nano3 Nh3 H2o Youtube

How To Write The Net Ionic Equation For Pb No3 2 Nh4cl Pbcl2 Nh4no3 Youtube

Net Ionic Equations Mrs Cummings Classes Home Pages 1 2 Flip Pdf Download Fliphtml5

How To Write The Net Ionic Equation For Nh4cl Naoh Nacl H2o Nh3 Youtube

Oneclass Write The Balanced Net Ionic Equation For The Reactions That Occur When The Following Aqueo

Solved Ionic Equation Worksheet Write The Balanced Equation Total Ionic Equation Ard Net Ionic Equation For The Reaction Ifany That Occurs When The Following Aqucous Solutions Ar Comnbined No Reaction Occurs Indicate With
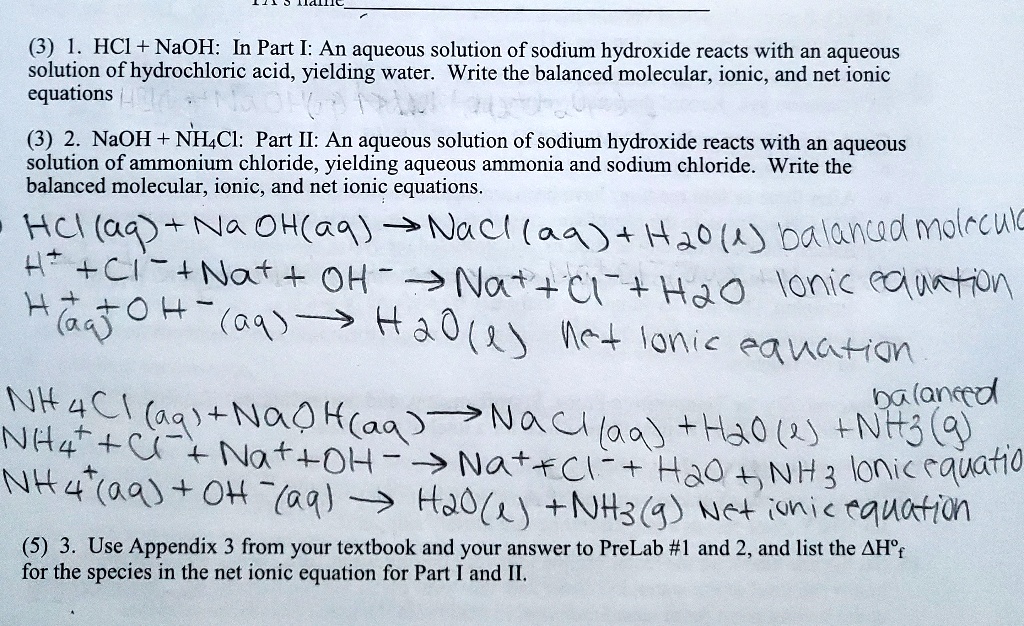
Solved 3 Hci Naoh In Part I An Aqueous Solution Of Sodium Hydroxide Reacts With An Aqueous Solution Of Hydrochloric Acid Yielding Water Write The Balanced Molecular Ionic And Net Ionic Equations
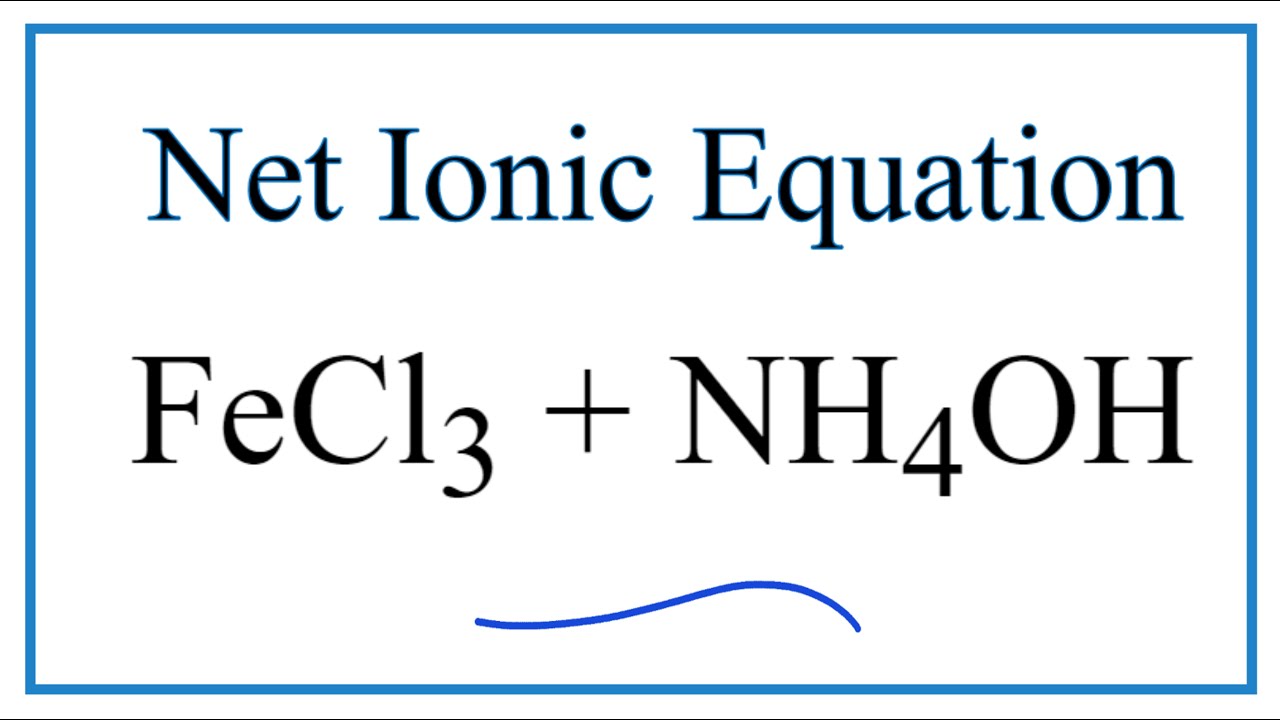
How To Write The Net Ionic Equation For Fecl3 Nh4oh Fe Oh 3 Nh4cl Youtube
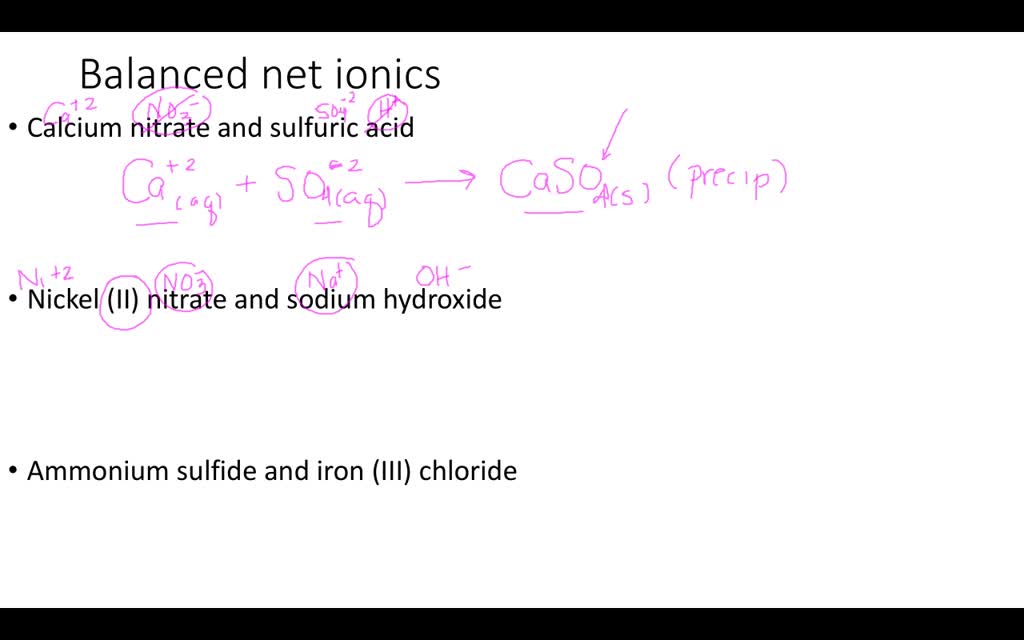
Solved Web Write A Balanced Net Ionic Equation For The Reaction Of Each Of The Following Aqueous Solutions With Mathrm Oh Ions A Ammonium Nitrate B Sodium Dihydrogen Phosphate Left Mathrm Nah 2 Mathrm Po 4 Right C Mathrm Al

Write The Net Ionic Equation When Aqueous Solutions Of Ammonium Carbonate And Iron Ii Nitrate Are Homeworklib

Solved 2 What Is The Net Ionic Equation When An Aqueous Chegg Com

Solved Write The Net Ionic Equation For The Reaction Of Chegg Com
Comments
Post a Comment